Deep Brain Stimulation (DBS) surgery is a medical procedure primarily used to treat neurological disorders, particularly those affecting movement. It involves implanting a device called a "neurostimulator" into specific areas of the brain. The device sends electrical impulses to targeted brain regions, helping to regulate abnormal brain activity.
Causes and Indications
DBS is most commonly used to treat movement disorders such as Parkinson's disease, essential tremor, and dystonia. It can also be considered for patients with certain psychiatric disorders like severe depression and obsessive-compulsive disorder (OCD), though this is less common. These conditions typically involve abnormal neural activity in specific brain regions, and DBS aims to alleviate symptoms by restoring normal functioning.
Symptoms Treated
Patients with Parkinson’s disease may experience tremors, rigidity, bradykinesia (slowness of movement), and postural instability. Essential tremor manifests as uncontrollable shaking, usually in the hands. Dystonia is marked by muscle contractions leading to twisting and abnormal postures. DBS helps reduce these symptoms, improving movement and quality of life. For psychiatric conditions like OCD and depression, DBS can help reduce severe symptoms when other treatments have failed.
Procedure
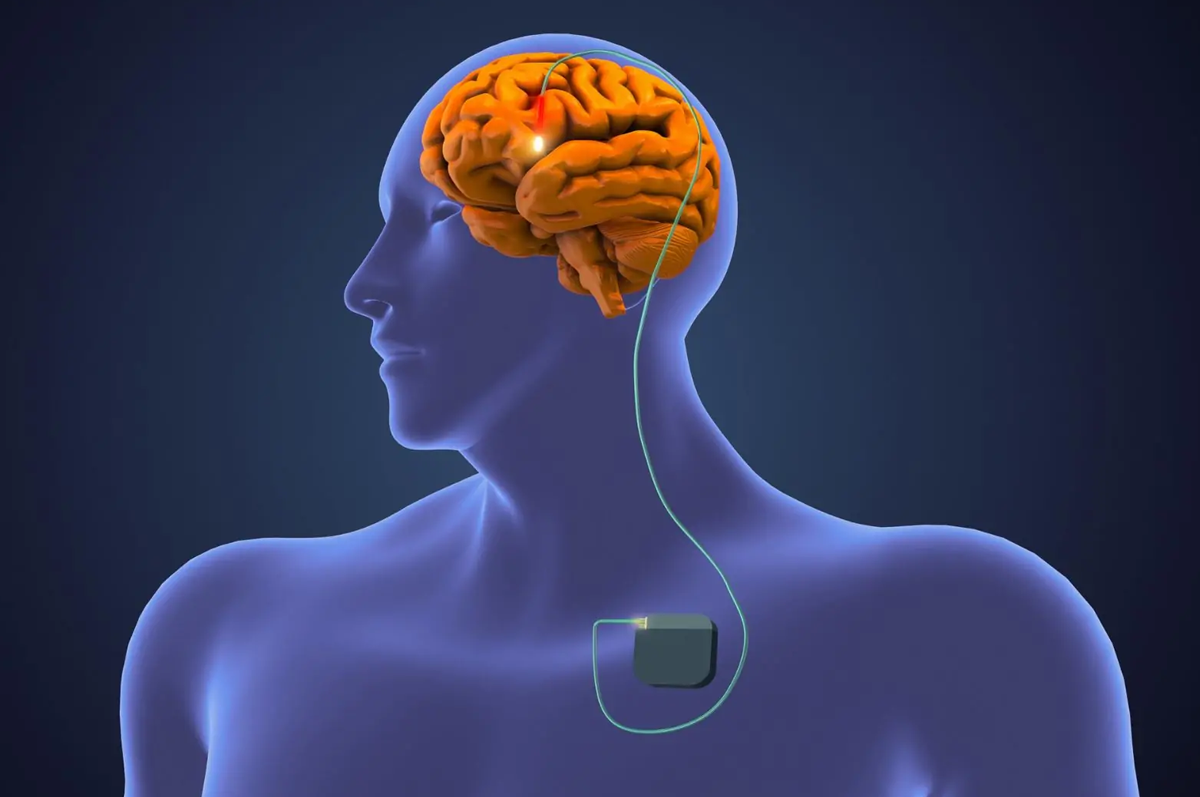
The DBS surgery is typically performed in two stages. First, electrodes are implanted into the targeted brain regions through small holes in the skull. These electrodes are connected to a pulse generator (similar to a pacemaker), which is implanted under the skin in the chest area. The device is then programmed to deliver electrical impulses to the brain. The settings can be adjusted over time to optimize symptom control.
Benefits and Risks
DBS can significantly reduce symptoms and improve the quality of life for many patients. However, it is not a cure, and it does not halt disease progression. Potential risks include infection, bleeding, device malfunction, or side effects like mood changes or cognitive issues. Regular follow-up is essential to monitor the device and adjust stimulation settings.